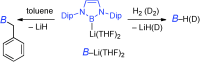(1) Chemistry of nucleophilic boryl anion
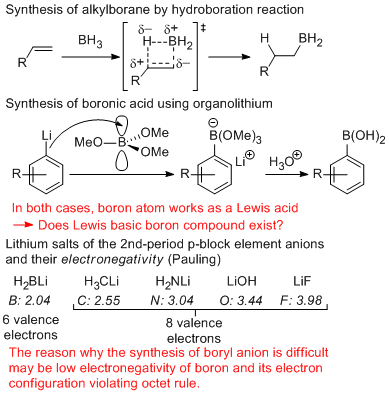
In organic chemistry, boron atom is known to show a variety of roles. The most of boron compounds
play as a Lewis acid in organic chemistry due to its vacant p-orbital to accept a lone pair electrons. Is there any nucleophilic boryl anion having a Lewis basicity?
Lithium salts of p-block element in the second period, such as alkyllithiums, lithium amides, lithium alkoxides, are widely used in synthetic organic chemistry because they can be purchased from chemical companies. However, the corresponding lithium salt of boryl anion is unknown compound. This elusive compound may allow us to utilize it as an equivalent of boryl anion and it may afford strong breakthrough in organic and inorganic chemistry.
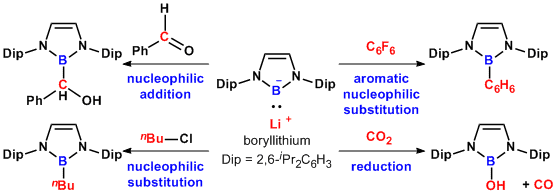
Recently, we have succeeded to synthesize and to isolate this boryllithium and revealed its interesting nature as a nucleophile. By X-ray crystallography and DFT calculation supported that the boron center in boryllithium would have nucleophilicity. In fact, boryllithium could add to benzaldehyde to form the corresponding adduct, alcohol. On the other hand, boryllithium could also substitute alkyl halides and electron-poor aryl halides. In the reaction with carbon dioxide, the resulting borylcarboxylate intermediate rearranged to release carbon monoxide with an oxidation at the boron center.
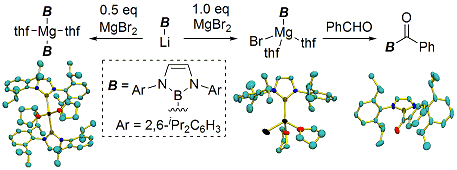
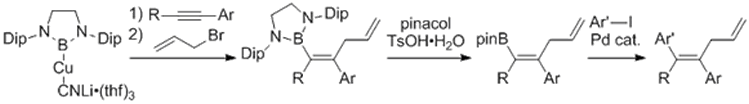
Salt metathesis reaction of boryllithium with magnesium, copper, and zinc afforded the corresponding borylmetal species having an anionic and nucleophilic character on the boron center. Especially, borylcopper species were shown to react with alkyne to produce carboborated products, which capable to undergo Suzuki-Miyaura cross coupling reaction for organic syntheses.
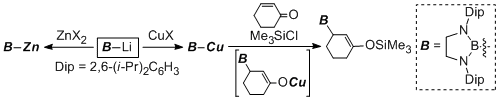
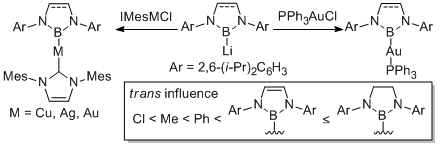
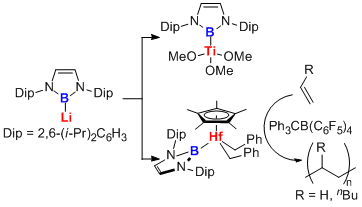
Transmetallation of boryllithium to transition metal halides could give the corresponding boryl transition metal complexes in easy manner. This methodology afforded the first syntheses of borylsilver, borylgold, boryltitanium, borylhafnium complexes. Among them, borylhafnium complex showed a catalytic acitivity for polymerization of ethylene and 1-hexene in the presence of co-catalyst.
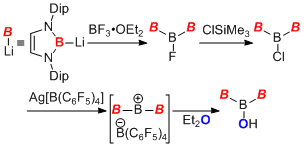
Nucleophilic borylation methodology using boryllithium is also effective for the preparation of novel boryl-substituted main group element compounds. Reaction of boryllithium with borane-THF complex easily gave the first example of boryltrihydroborate compound. It worked as a radical hydrogen donor probably due to its small bond dissociation energy for BH bond and high solubility of the compounds in hydrocarbon solvent. On the other hand, reaction of boryllithium with BF3-OEt2 complex afforded a triborane derivative having a linear topology. In the boron chemistry, this kinds of catenated boron compounds are rare and this is the second example.
Due to the lower electronegativity of boron atom than that of carbon atom, it can be expected that boryl anion could have higher basicity compared to that of carbanion. In fact, treatment of boryllithium with toluene led to deprotonation of methyl group of toluene. Additionally, it was also found that boryl anion could deprotonate H2 molecule to give lithium hydride and hydroborane. This deprotonation took place more rapidly than that of deprotonation of H2 with alkyllithium to show high basicity of boryl anion.
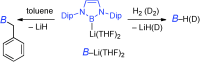
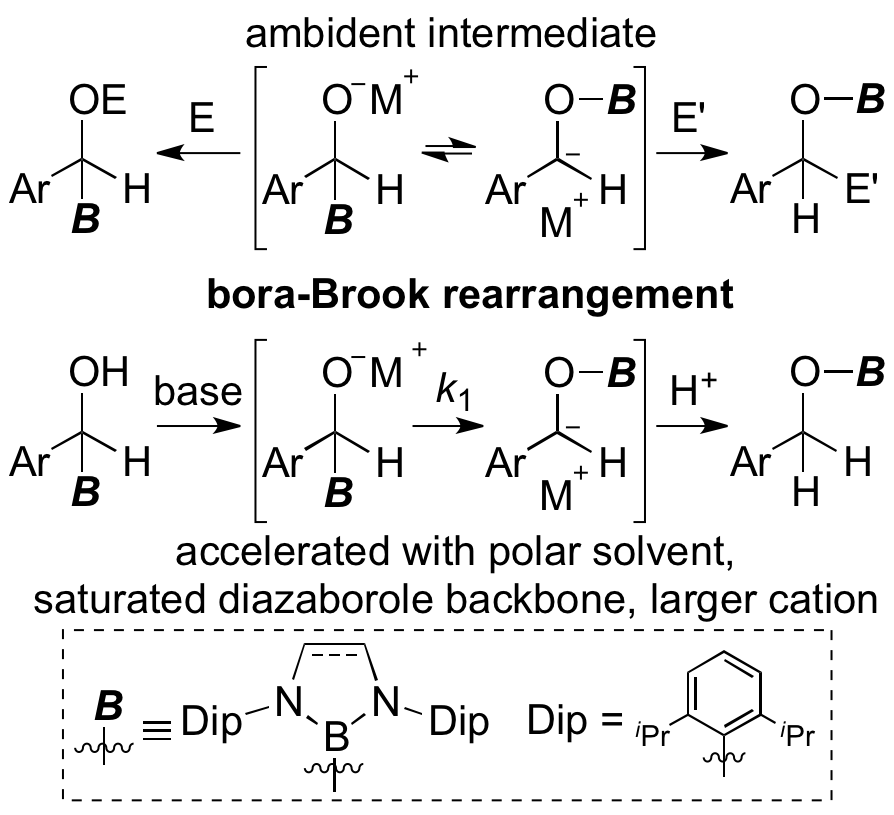
α-Borylbenzyul alcohol derived from boryl anion and benzealdehyde was deprotonated to induce a rearrangement of boryl group from carbon atom to oxygen atom. This rearrangement reaction proceeded through a similar mechanism of the Brook rearrangement which is popular in silicon chemistry. The reaction kinetics also revealed acceleration effect with larger alkali metal cation, coordinating solvent, and boryl group possessing saturated skeleton.
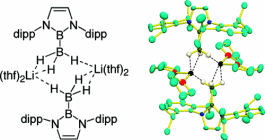
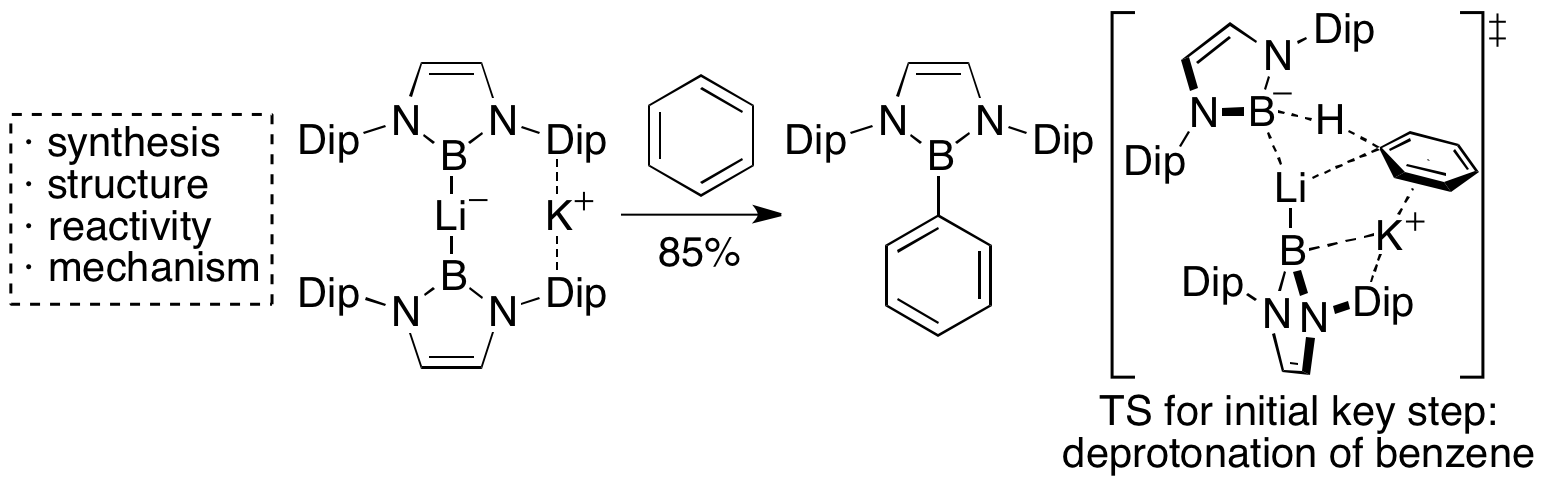
Bromoborane precursor for boryllithium was reduced with Li and Na/K alloy to give diboryllithate complex in which two broyl anions coordinate to lithium cation. This compound exhibited very low-field shifted 7Li NMR signal due to the existence of two N-B-N unit having π-electrons. This compound could directly deprotonate benzene to show higher basicity than that of boryllithium.
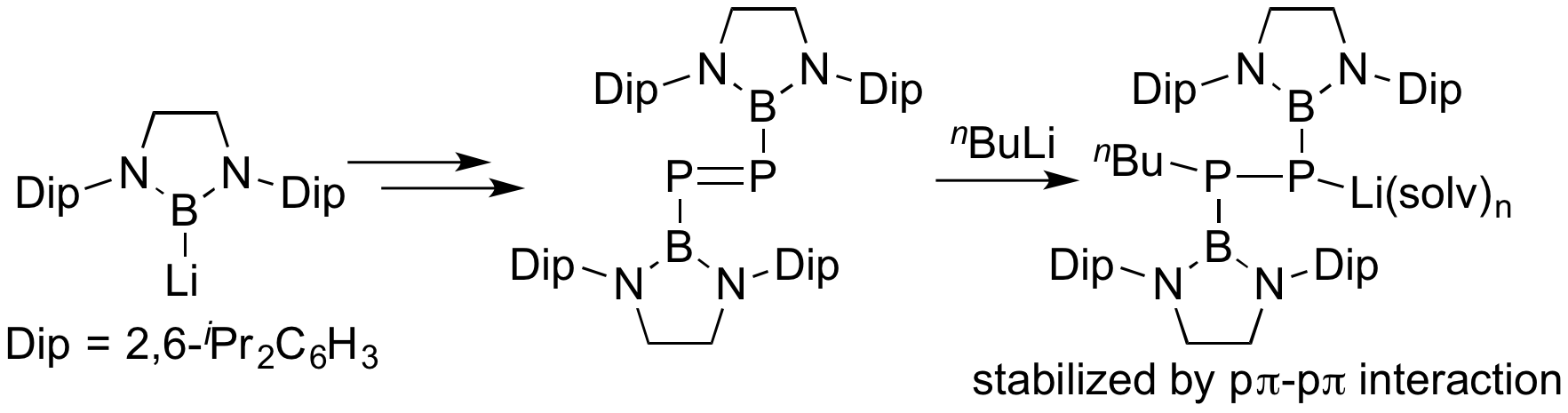
Boryl anion could also be used for the synthesis of boryl-substituted main group element compounds. Borylzinc species generated by transmetallation of boryllithium reacted with PCl3 followed by reduction with Mg to produce boryl-substituted diphosphene, P=P double bond species. Reaction of the previously reported Mes*-substituted diphosphene with nBuLi afforded an unstable adduct, while the reaction of boryl-substituted diphosphene with nBuLi formed stable adduct due to π-accepting character of boryl substituent.
Related publications
(1) Segawa, Y.; Yamashita, M.; Nozaki, K. Science 2006, 314, 113-115. This article was highlighted in Science, Chemistry World, and C&EN.
(2) Yamashita, M.; Suzuki, Y.; Segawa, Y.; Nozaki, K. J. Am. Chem. Soc. 2007, 129, 9570-9571. doi.This article was highlighted in Nachrichten aus der Chemie.
(3) Segawa, Y.; Yamashita, M.; Nozaki, K. Angew. Chem. Int. Ed. 2007, 46, 6710-6713. doi. This article was highlighted in Nachrichten aus der Chemie.
(4) Segawa, Y.; Suzuki, Y.; Yamashita, M.; Nozaki, K. J. Am. Chem. Soc. 2008, 130, 16069-16079. doi
(5) Yamashita, M.; Suzuki, Y.; Segawa, Y.; Nozaki, K. Chem. Lett.2008, 37, 802-803. doi
(6) Kajiwara, T.; Terabayashi, T.; Yamashita, M.; Nozaki, K. Angew. Chem. Int. Ed. 2008, 47, 6606-6610. doi
(7) Terabayashi, T.; Kajiwara, T.; Yamashita, M.; Nozaki, K. J. Am. Chem. Soc. 2009, 131, 14162-14163. doi
(8) Nozaki, K.; Aramaki, Y.; Yamashita, M.; Ueng, S.-H.; Malacria, M.;
Lacote, E.; Curran, D. J. Am. Chem. Soc. 2010, 132, 11449-11151. doi
(9) Okuno, Y.; Yamashita, M.; Nozaki, K. Angew. Chem. Int. Ed. 2011, 50, 920-923. doi
(10) Hayashi, Y.; Segawa, Y.; Yamashita, M.; Nozaki, K. Chem. Commun. 2011, 47, 5888-5890. doi
(11) Okuno, Y.; Yamashita, M.; Nozaki, K. Eur. J. Org. Chem., 2011, 3951-3958. doi
(12) Dettenrieder, N.; Aramaki, Y;. Wolf, B.; Maichle-Mössmer, C.; Zhao, X.; Yamashita, M.; Nozaki, K.; Anwander, R. Angew. Chem. Int. Ed. 2014, 53, 6259-6262. doi
(13) Kisu, H.; Sakaino, H.; Ito, F.; Yamashita, M.; Nozaki, K. J. Am. Chem. Soc. 2016, 138, 3548-3552. doi
(14) Ohsato, T.; Okuno, Y.; Ishida, S.; Iwamoto, T.; Lee, K.-H.; Lin, Z.; Yamashita, M.; Nozaki, K. Angew. Chem. Int. Ed. 2016, 55, 11426-11430. doi
(15) Asami, S.-s.; Okamoto, M.; Suzuki, K.; Yamashita, M. Angew. Chem. Int. Ed. 2016, 55, 12827-12831. doi
(16) Asami, S.-s.; Ishida, S.; Iwamoto, T.; Suzuki, K.; Yamashita, M. Angew. Chem. Int. Ed. 2017, 56, 1658-1662. doi
(17) Asami, S.-s.; Suzuki, K.; Yamashita, M. Chem. Lett. 2017, 46, 686-689. doi
(18) Yagi, A.; Kisu, H.; Yamashita, M. Dalton Trans. 2019, 48, 5496-5499. doi
(19) Kisu, H.; Kosai, T.; Iwamoto, T.; Yamashita, M. Chem. Lett. 2021, 50, 293-296. doi