2-12-1 Ookayama, Meguro-ku, Tokyo 152-8551, Japan
Department of Chemistry,
School of Science, Institute of Science Tokyo
Access to Ookayama campus, campus map
East 1 Building,
room 41, 44, 45
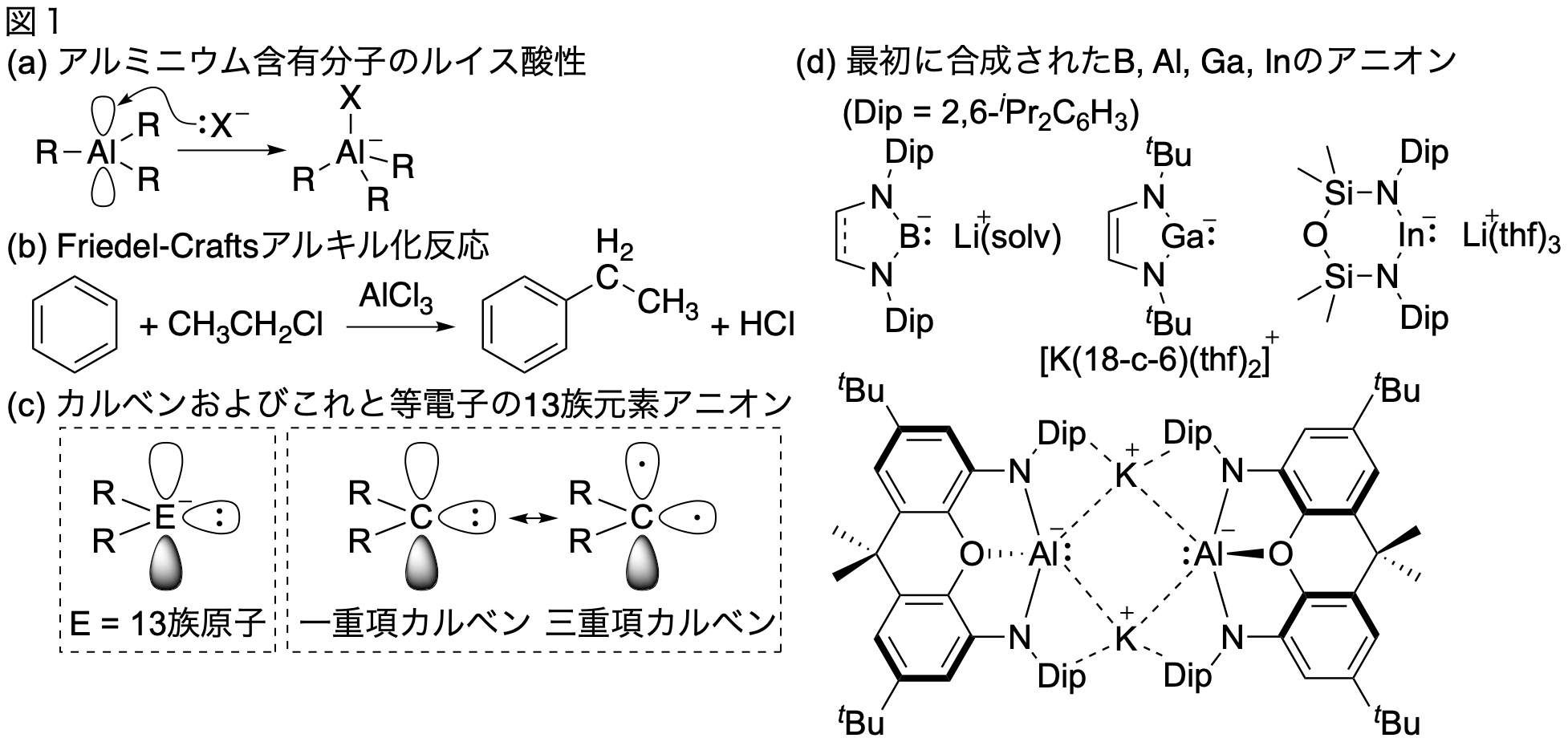
Al is not only widely used as its elemental form, such as aluminum foil, aluminum cans, and 1-yen coins around us, but also as a compound such as alloys and alumina/zeolite. THat is, Al is an element that widely supports our lives. As described above, while the application as an inorganic chemical material utilizing the electrically positive (= high ionization potential) property of Al is widely used, there is also a chemistry using an aluminum-containing molecule. Since Al is a Group 13 element and has three valence electrons, a neutral Group 13 element-containing molecule will have three bonds and one empty orbital, and will accept electron pairs from other species as a Lewis acid [Fig. 1 (a)]. For this reason, Al-containing molecules are widely used as a catalyst utilizing its Lewis acidity in polymer synthetic chemistry and organic synthetic chemistry. Some of readers for this text may remember that AlCl3 is used as a catalyst in the "Friedel-Crafts alkylation of benzene [Fig. 1 (b)]" in an undergraduate course of organic chemistry. On the other hand, since aluminum is electrically positive, aluminum compounds having a negative charge and an unshared electron pair and exhibiting Lewis basicity are rare. In organic chemistry, carbene is a species having two bonds, and is known as a highly reactive intermediate. There are a total of 6 valence electrons around the carbene carbon, and the central carbon atom has two orbitals and two electrons in addition to two covalent bonds of two centers and two electrons [Fig. 1]. (c)]. When these two non-bonded electrons are in the same orbital, it is singlet carbene, and when they are in different orbitals, it is triplet carbene. On the other hand, a 6-electron species with an isoelectronic structure in which carbene carbon is replaced with a group 13 element will have an anionic charge. Until recently, the chemistry of this group 13 element anion was limited to B- (Segawa, Y.; Yamashita, M.; Nozaki, K., Science 2006, 314, 113-115.) and Ga- (Schmidt, E. S.; Jockisch, A.; Schmidbaur, H., J. Am. Chem. Soc. 1999, 121, 9758-9759; Baker, R. J.; Farley, R. D.; Jones, C.; Kloth, M.; Murphy, D. M., J. Chem. Soc., Dalton Trans. 2002, 3844-3850.) centered anions. Examples of Al- and In- anions were reported one after another in 2018 and 2019 [Fig. 1 (d), Hicks, J.; Vasko, P.; Goicoechea, J. M.; Aldridge, S., Nature 2018, 557, 92-95; Schwamm, R. J.; Anker, M. D.; Lein, M.; Coles, M. P., Angew. Chem. Int. Ed. 2019, 58, 1489-1493.]. Since all Group 13 element anions are singlet and exhibit Lewis basicity and nucleophilicity, these are contrary to the common wisdom that "Group 13 element-containing molecules exhibit Lewis acidity derived from their empty orbitals". The key to successful isolation of these highly reactive species would be an introduction of the electronegative nitrogen substituents on the group 13 element to stabilize their negative charge. In contrast, we recently found that an Al anion 1, which has only an alkyl group, can be synthesized. The details will be described below.
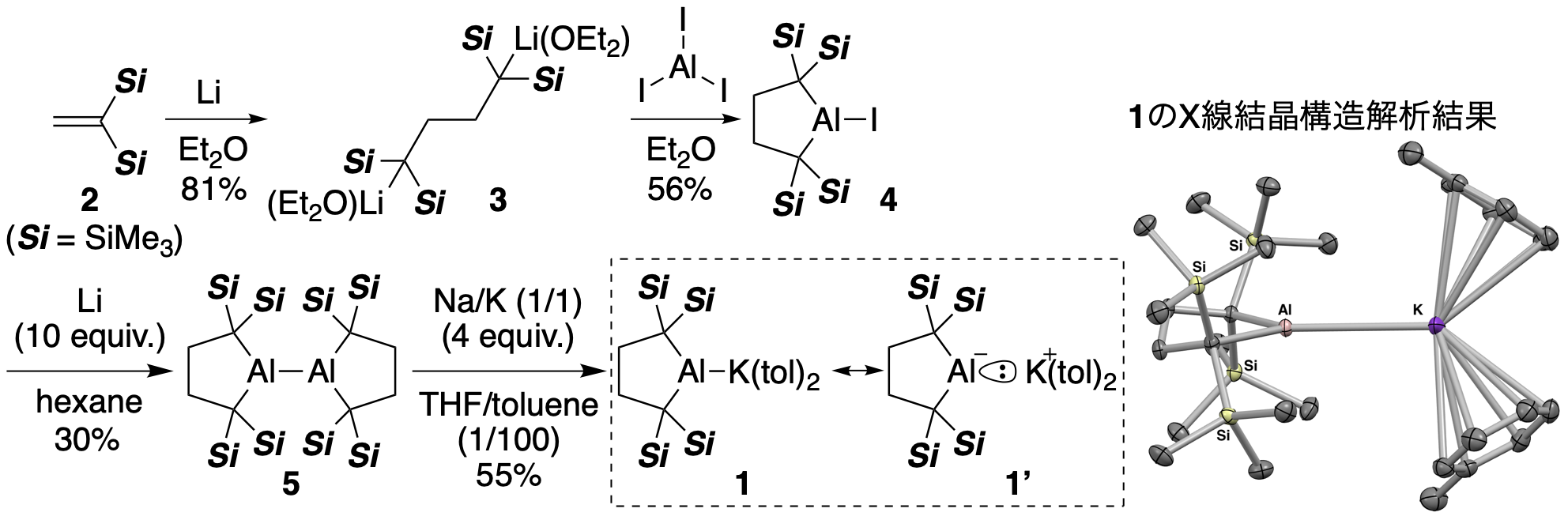
Al anion 1 was synthesized by the route shown below. By a reaction of bis(trimethylsilyl)ethylene 2 with elemental Li in diethyl ether, the reductive coupling reaction proceeds to obtain dilithiobutane 3 in which a C–C bond is formed. It is reported by Kira and Iwamoto of Tohoku University (Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C., J. Am. Chem. Soc. 1999, 121, 9722.) that the same reaction can be carried out in THF to synthesize a similar dianion equivalent and subsequent cyclization of it with silicon affords a divalent and dicoordinated silicon compound silylene. When aluminum triiodide reacted with 3, the nucleophilic substitution reaction proceeded twice on the Al atom to furnish cyclic iodoalumane 4. Treatment of 4 with elemental Li gave dialumane 5 with an Al–Al bond through reductive cleavage of the Al–I bond followed by dimerization of the Al radical intermediate. When 5 reacted with a Na/K alloy in a mixed solvent of THF/toluene, the Al–Al bond was reductively cleaved to obtain 1 having an Al–K bond. Single crystal X-ray crystallography revealed that 1 crystallized with 2 molecules of toluene used as the solvent. 1 had an unprecedented Al–K single bond with a bond length of 3.4549(5) Å (Table 1). Considering the electronegativity of each atom, since this Al–K bond is polarized to Al–K+, it is considered that the Al atom has a contribution of resonance structure 1' having a lone pair of electrons. The Al–C bond [2.0846(9) Å] of 1 is clearly longer than those of the precursor 5 [2.005(3)–2.011(3) Å], and the C–Al–C bond angle [90.40 (5)°] in 1 was smaller than that of 5 [97.78(12)°]. A tetrahedral molecule, represented by the methane, has a bond angle of about 109.5 °, which uses an sp3 hybrid orbital in which one s orbital of carbon and three orthogonal p orbitals are mixed to make a bond with hydrogen. If a bond is formed here by using only p-orbitals which are orthogonal to each other, the C–Al–C bond angle should be 90°. That is, when comparing the above 1 and the precursor 5, the difference in structure that 1 has a bond angle closer to 90° explains that the component of p-orbitals of the Al atom in the Al–C bond of 1 is higher than that of 5. From a different point of view, it can be said that the structure around the Al atom changed in the reaction generated from 5 to 1 in order to accommodate the unshared electron pair of the Al atom of 1 in the orbital having a high component of s-orbitals. In addition, 1 is a compound having π-electrons only on the toluene molecule coordinated to the K+ ion, but showed an absorption in the visible light region (309, 468 nm, toluene, -50 °C).
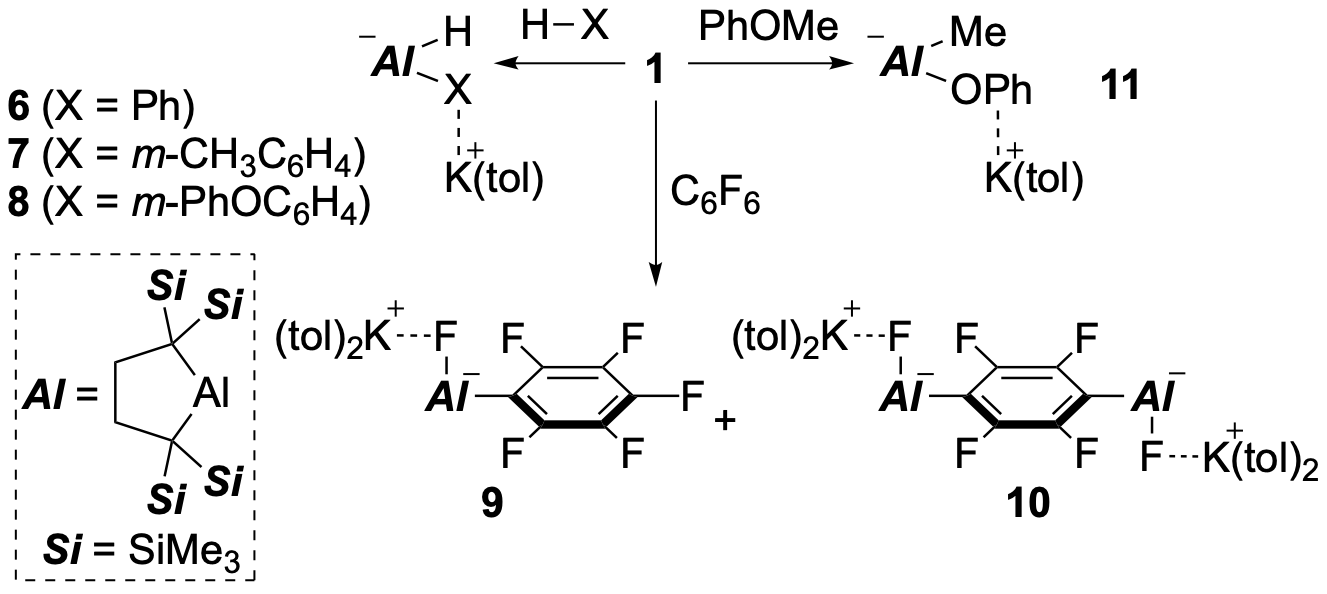
Dialkyl Al anion 1 gives (hydro)(phenyl)aluminate 6 by reaction with benzene. Similarly, 1 reacted with toluene and diphenyl ether to give 7, 8 with the selective oxidative addition of the C–H bond at the meta position. It is determined by DFT calculations that the reaction between 1 and toluene proceeds through an aromatic nucleophilic substitution type reaction mechanism. In the reaction between 1 and C6F6, the aromatic nucleophilic substitution reaction proceeds in the same manner, and monoaluminated product 9 and dialuminated product 10 are obtained. At this time, even if the stoichiometry of C6F6 to 1 is increased to 10 equivalents, the dialuminated product 10 is still produced. Therefore, it is considered that the second alumination to C6F6 is faster than the first alumination. On the other hand, the reaction between 1 and anisole yields product 11 with an oxidative addition of a C–O bond, in which the Al atom nucleophilically attacks to the Me group and the resulting phenoxide ion subsequently attacks to the Al atom.
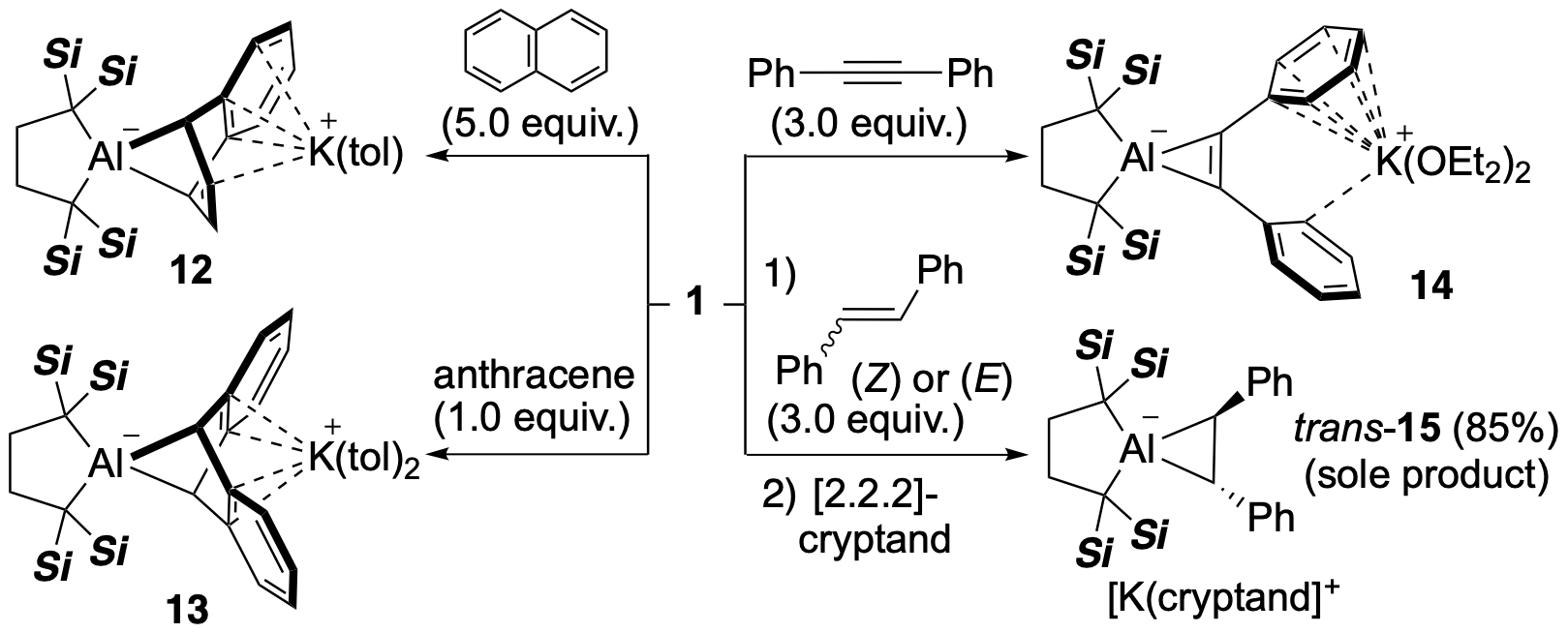
It was also found that 1 undergoes a cycloaddition reaction with unsaturated hydrocarbons. When 1 was mixed with naphthalene or anthracene, the (1+4) cycloaddition reaction proceeded, and aluminum-containing norbornadiene derivatives 12 and 13 were obtained. The structure of each product has been confirmed by X-ray crystallography. In the reaction of 1 with diphenylacetylene, the (1+2) cycloaddition reaction proceeded, and aluminacyclopropene 14 was obtained. The reaction of 1 with (E)- or (Z)-stilbene also proceeded through the (1+2) cycloaddition reaction, but the same trans adduct trans-15 was produced in both cases. According to the mechanistic study using computational chemistry, the reactions of 1 with (E)- or (Z)-stilbene proceeded in a concerted one-step reaction, but in the case of (Z)-stilbene, a stereochemical inversion took place during the conversion from the carbanionic transition state to the three-membered ring. As described above, since Al anion 1 should be considered as Al(I), these reactions can be considered as an "oxidative" cycloaddition to give an Al(III) compound.
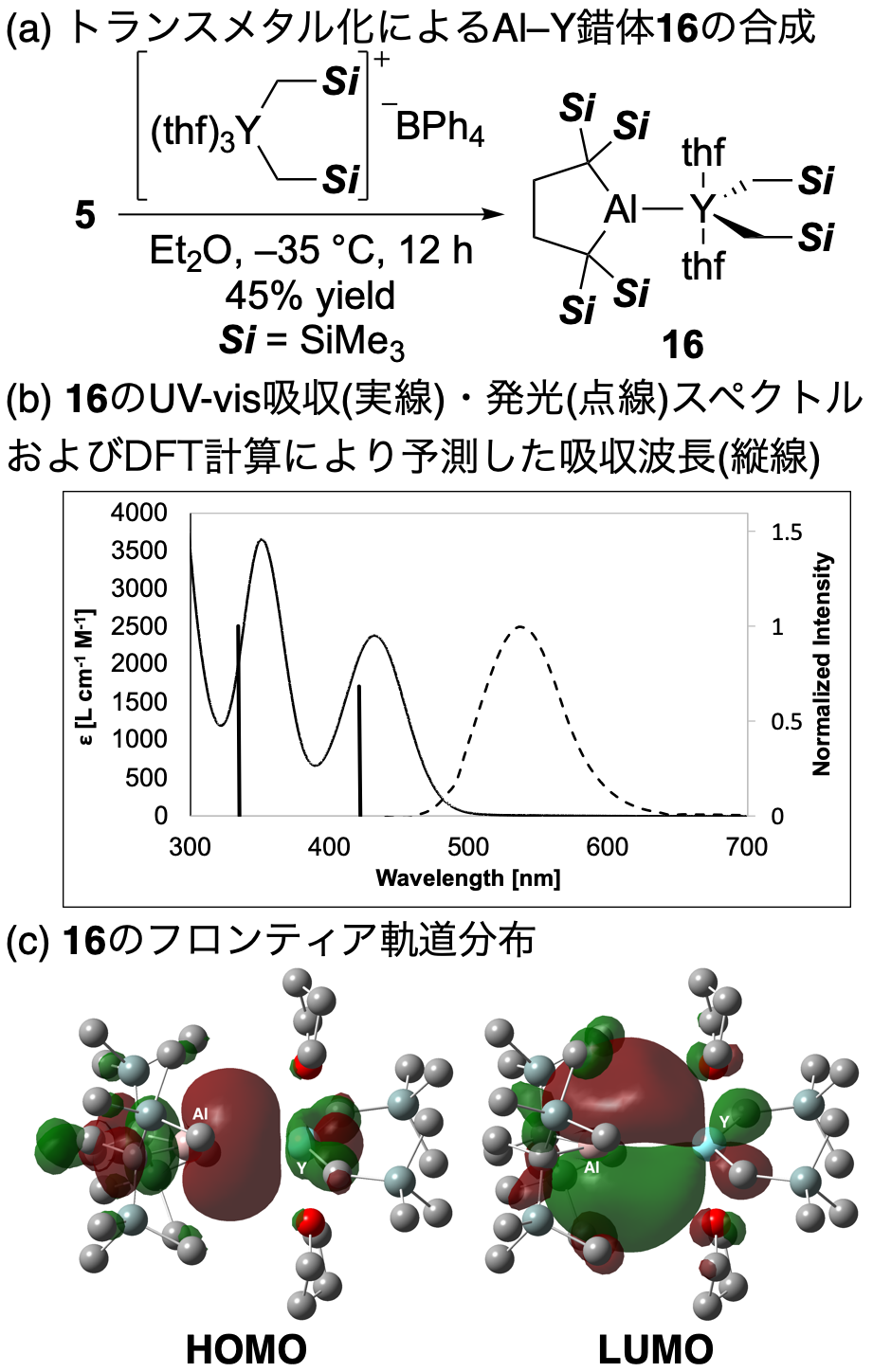
It was also clarified that Al anion 1 undergoes transmetallation to a transition metal complex. Reaction of 1 with the cationic dialkylyttrium complex [Y(CH2SiMe3)2(thf)3][BPh4] gave complex 16 with an unprecedented Al–Y single bond as bright yellow crystals. The 1H NMR spectrum of complex 16 shows that it has a C2v-symmetrical molecular structure. Since the carbon atom adjacent to the Al atom appeared as a characteristic doublet (2JYC = 6 Hz) in the 13C NMR spectrum, it became clear that the Al–Y single bond was retained in solution. By X-ray crystallography, two independent molecules of 16 were observed in the unit cell, and their Al–Y bond lengths were 3.1870(8) and 3.1942(8) Å. Since these are longer than the sum of the covalent radii of 2.87 Å, indicating the high ionic character of the Al–Y bond, and AIM analysis also supports its polarization. Because complex 16 exhibited a yellow color, the absorption spectrum was measured to show maximum absorptions at 351 nm (ε = 3700) and 432 nm (ε = 2400). From the TD-DFT calculations, it was also found that the transition at the longer wavelength corresponds to the HOMO-LUMO transition. Here, the Al–Y bond, whose orbital energy has been raised due to the highly electron-donating property of the Al anion, corresponds to HOMO, and a characteristic LUMO which consists of the empty p-orbital of the Al atom and the empty d-orbital of the Y atom was observed. That is, the electronic characteristics of the Al anion ligand made the HOMO-LUMO energy gap of the d0 metal complex be narrow with no π-electrons and no d-d transition to have absorption in the visible light region. It was also found that 16 emits light at 536 nm (φ = 0.0016), although it is weak.